Lewis Dot Structure For Scl4
I'one thousand super excited to teach you lot the lewis structure of SCl4 molecule in just v unproblematic steps.
Infact, I've also given the step-past-pace images for drawing the lewis dot structure of SCl4 molecule.
So, if you are ready to go with these five simple steps, and so let's dive right into it!
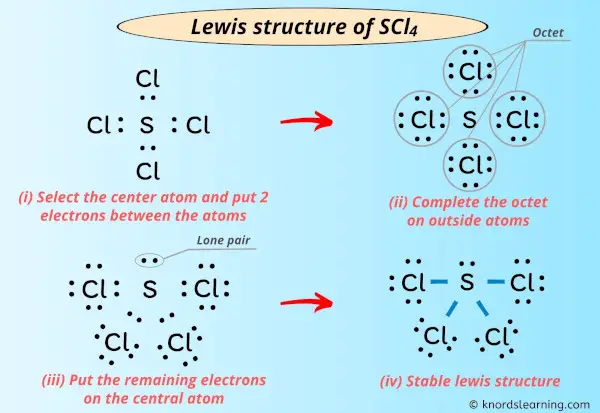
Lewis construction of SCl4 contains four unmarried bonds between the Sulfur (S) atom and each Chlorine (Cl) atom. The Sulfur atom (Due south) is at the center and information technology is surrounded past iv Chlorine atoms (Cl). The Sulfur cantlet has one lone pair while all the four chlorine atoms have 3 lone pairs.
Permit's draw and sympathise this lewis dot structure stride by step.
(Note: Take a pen and newspaper with yous and try to describe this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of SCl4).
5 Steps to Draw the Lewis Structure of SCl4
Step #1: Summate the full number of valence electrons
Hither, the given molecule is SCl4. In social club to describe the lewis structure of SCl4, first of all you have to find the total number of valence electrons nowadays in the SCl4 molecule.
(Valence electrons are the number of electrons nowadays in the outermost shell of an atom).
So, let'due south calculate this start.
Adding of valence electrons in SCl4
- For Sulfur:
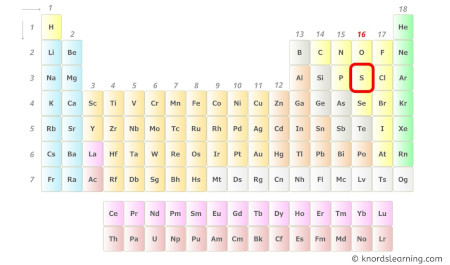
Sulfur is a group sixteen element on the periodic table.
Hence, the valence electrons present in sulfur is 6 (see beneath image).

- For Chlorine:
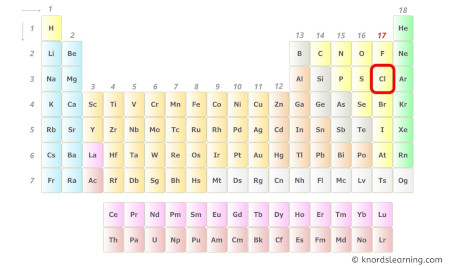
Chlorine is a grouping 17 element on the periodic table.
Hence, the valence electron nowadays in chlorine is 7 (encounter beneath epitome).
Hence in a SCl4 molecule,
Valence electrons given by Sulfur (S) atom = 6
Valence electrons given by each Chlorine (Cl) cantlet = 7
And so, total number of Valence electrons in SCl4 molecule = vi + 7(4) = 34
Step #2: Select the eye cantlet
While selecting the atom, always put the to the lowest degree electronegative atom at the center.
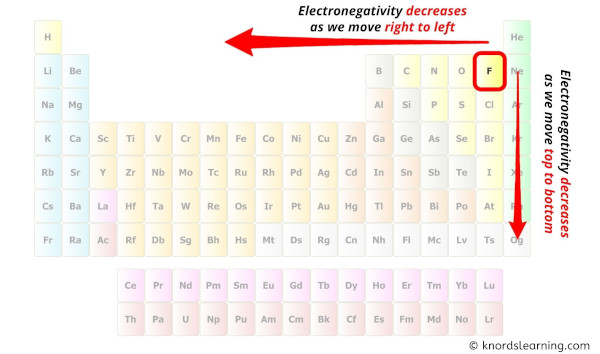
(Remember: Fluorine is the most electronegative chemical element on the periodic table and the electronegativity decreases as we move correct to left in the periodic tabular array as well as meridian to bottom in the periodic table).
Here in the SCl4 molecule, if nosotros compare the sulfur atom (S) and chlorine atom (Cl), then the sulfur is less electronegative than chlorine.
And so, sulfur should be placed in the center and the remaining 4 chlorine atoms will surround information technology.
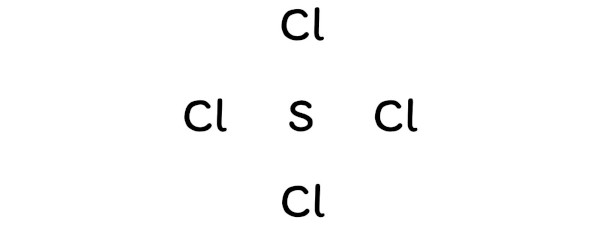
Step #3: Put ii electrons between the atoms to represent a chemic bond
Now in the above sketch of SCl4 molecule, put the two electrons (i.e electron pair) between each sulfur atom and chlorine atom to represent a chemical bond between them.
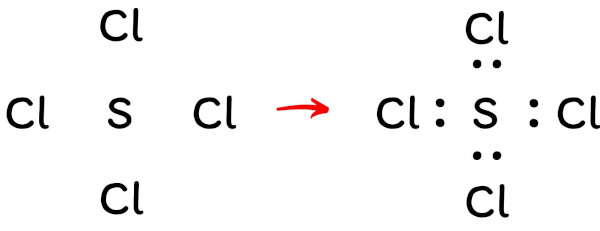
These pairs of electrons present betwixt the Sulfur (South) and Chlorine (Cl) atoms form a chemical bond, which bonds the sulfur and chlorine atoms with each other in a SCl4 molecule.
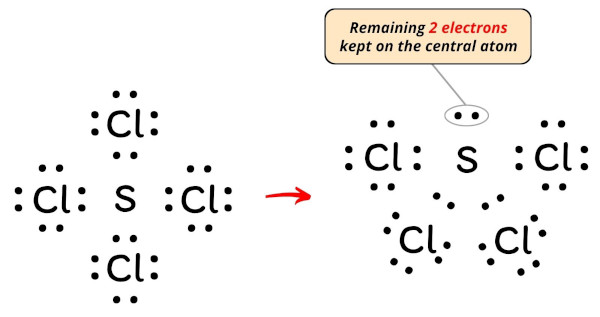
Stride #4: Consummate the octet (or duplet) on outside atoms. If the valence electrons are left, then put the valence electrons pair on the cardinal cantlet
Don't worry, I'll explain!
In the Lewis construction of SCl4, the outer atoms are chlorine atoms.
Then now, you accept to complete the octet on these chlorine atoms (considering chlorine requires 8 electrons to have a consummate outer shell).
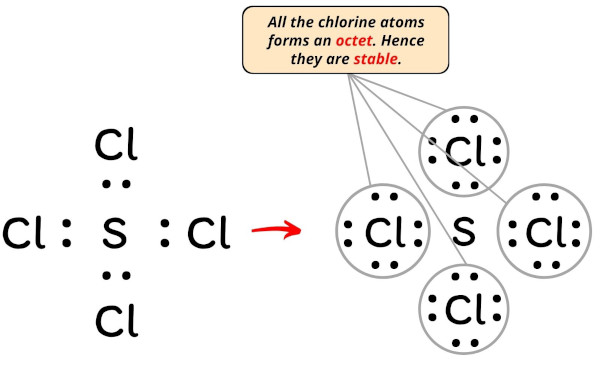
Now, y'all can see in the in a higher place paradigm that all the chlorine atoms form an octet.
Besides, simply 32 valence electrons of SCl4 molecule are used in the in a higher place construction.
Merely there are total 34 valence electrons in SCl4 molecule (every bit calculated in stride #1).
Then the number of electrons left to exist kept on the central atom = 34 – 32 = ii.
So let's keep these two electrons (i.e electron pair) on the central cantlet.
Now, let'south movement to the next step.
Step #5: Terminal pace – Check the stability of lewis structure past calculating the formal accuse on each atom
Now, you take come to the final step and here you have to check the formal accuse on sulfur atom (S) as well as each chlorine atom (Cl).
For that, y'all demand to recollect the formula of formal charge;
Formal charge = Valence electrons – Nonbonding electrons – (Bonding electrons)/2
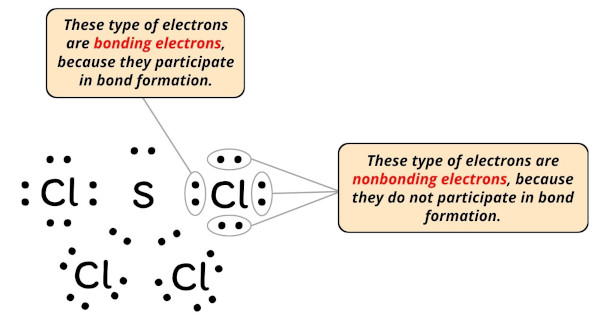
- For Sulfur:
Valence electrons = 6 (every bit it is in group 16)
Nonbonding electrons = 2
Bonding electrons = 8 - For Chlorine:
Valence electron = seven (every bit information technology is in group 17)
Nonbonding electrons = 6
Bonding electrons = 2
| Formal charge | = | Valence electrons | – | Nonbonding electrons | – | (Bonding electrons)/two | ||
| S | = | half-dozen | – | 2 | – | 8/ii | = | 0 |
| Cl | = | 7 | – | 6 | – | ii/two | = | 0 |
So you can come across higher up that the formal charges on sulfur as well as chlorine are "zip".
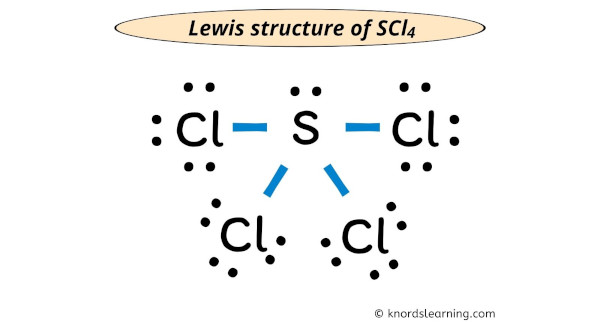
Hence, there will non be any change in the above construction and the above lewis structure of SCl4 is the final stable structure only.
Each electron pair (:) in the lewis dot structure of SCl4 represents the single bond ( | ). And so the above lewis dot structure of SCl4 can besides be represented as shown below.
Related lewis structures for your practice:
Lewis Structure of PBr5
Lewis Structure of SiS2
Lewis Structure of OCS
Lewis Structure of Br3-
Lewis Structure of H3O+
Lewis Dot Structure For Scl4,
Source: https://knordslearning.com/lewis-structure-of-scl4/
Posted by: millswhimen.blogspot.com


0 Response to "Lewis Dot Structure For Scl4"
Post a Comment